Contact Information
PI: Judith K. Brown - School of Plant Sciences, University of Arizona, Tucson, AZ 85721
Email: jkbrown@arizona.edu
Co-PI: Jawwad A. Qureshi - Department of Entomology and Nematology, University of Florida, Southwest Florida Research and Education Center, Immokalee, FL 34142 Email: jawwadq@ufl.edu
Overview of the Problem
Citrus greening disease
Citrus greening or Huanglongbing is among the most economically-important citrus diseases worldwide and has contributed substantially to economic losses incurred by the citrus industry.

Symptoms of citrus greening disease
Symptoms of citrus greening disease are manifest as blotchy mottling of leaves, ‘chlorosis’ or yellowing of the new shoots, and tree dieback, eventually followed by death. Fruits are discolored, pithy, and lower than normal in sugar content. The citrus fruits become increasingly unmarketable as the disease progresses (Bové 2006; Da Graça 1991).
(click on photos below for expanded view)
Causal agent of citrus greening disease
Citrus greening disease is associated with three phloem-limited alpha-proteobacteria including ‘Candidatus Liberibacter asiaticus’ (CLas), ‘Ca. L. americanus’, and ‘Ca. L. africanus’, with origins in Asia, Brazil and Africa, respectively (Bové 2006; Teixeira et al. 2005). The most prevalent and widespread species is CLas, and disease associated with the disease found in Asia and the Americas. All known species of Liberibacter are known to be transmitted from tree to tree by grafting of contaminated citrus stocks plants, and by short and long distance by one of several species of psyllid vector.
The Asian citrus psyllid (ACP), Diaphorina citri Kuwayama (Hemiptera: Liviidae) is the most important vector of citrus greening disease because it is prevalent in most citrus-growing regions of the world. Management of citrus greening disease relies on planting of clean stock and nursery stock, psyllid vector control and tree removal programs, however, current practices have been insufficient to abate the continued spread of the disease, once established.

Citrus greening disease caused by “Ca. Liberibacter asiaticus” is transmitted by the Asian citrus psyllid (Diaphorina citri)
David Hall, USDA-ARS
Transmission of CLso by ACP adults occurs in a persistent, propagative manner, and CLso cells can be observed throughout the ACP midgut, hemocoel, fat body, salivary glands, and ovaries of CLas-infected psyllids. Transmission is most efficient when early-instar nymphs ingest the bacterium from citrus leaves on which eggs are oviposited during female feeding. Upon molting to adult-stage, psyllids are competent for transmission throughout their adult lives. After ingestion, CLas accumulates to increasingly greater numbers over the course of the infection cycle, with the highest relative titer detectable in the fourth immature instar and young (teneral) adults. Acquisition of CLas by adult ACP does not typically result in competent transmission.
Management of citrus greening disease relies on planting of clean stock and nursery stock, psyllid vector control and tree removal programs, however, current practices have been insufficient to abate the continued spread of the disease, once established.
Project Overview
In previous studies, we and other researchers have demonstrated that RNAi effectively reduces or ‘silences’ the expression of selected gut gene targets in psyllids, resulting in psyllid mortality. Recently, a second class of targets have been silenced and shown to alter Liberibacter accumulation (relative titer) in the psyllid vector host, potentially interfering with invasion and/or establishment in the gut, with several showing promise for reducing potato psyllid-mediated CLso transmission.
Further, our results have shown that by silencing 2-5 targets at the same time, or 'stacking' may multiply the effects of RNAi. Stacking has the further added benefit that psyllids will be unlikely to develop resistance to 'therapeutic' dsRNA treatment. Thus, dsRNA therapy offers a highly target-specific, non-toxic (humans, off-target organisms, and environmental) that does not require GMO technology for effective delivery of an effective dose to the plant, and will control CLas and ACP simultaneously, leading to reduced psyllid population size and lowered CLas levels in citrus trees.
Several groups have demonstrated the utility of light laser technology for creating tiny wounds in the epidermis of citrus leaves to enable entry of dsRNA into the vasculature. In this study, we are furthering those observations to investigate the potential of different surfactants, penetrants, and protectants for enabling dsRNA directly into the leaves following laser-wounding. Several types of lasers have been evaluated for similar laboratory and field applications, including a model designed for use in citrus groves to enable foliar delivery of small molecules and compounds. In this project, the efficacy of laser-mediated delivery of dsRNA into citrus trees will be evaluated. Together, laser-mediated or other types of foliar delivery of dsRNA are expected to offer important new tools for HLB management.
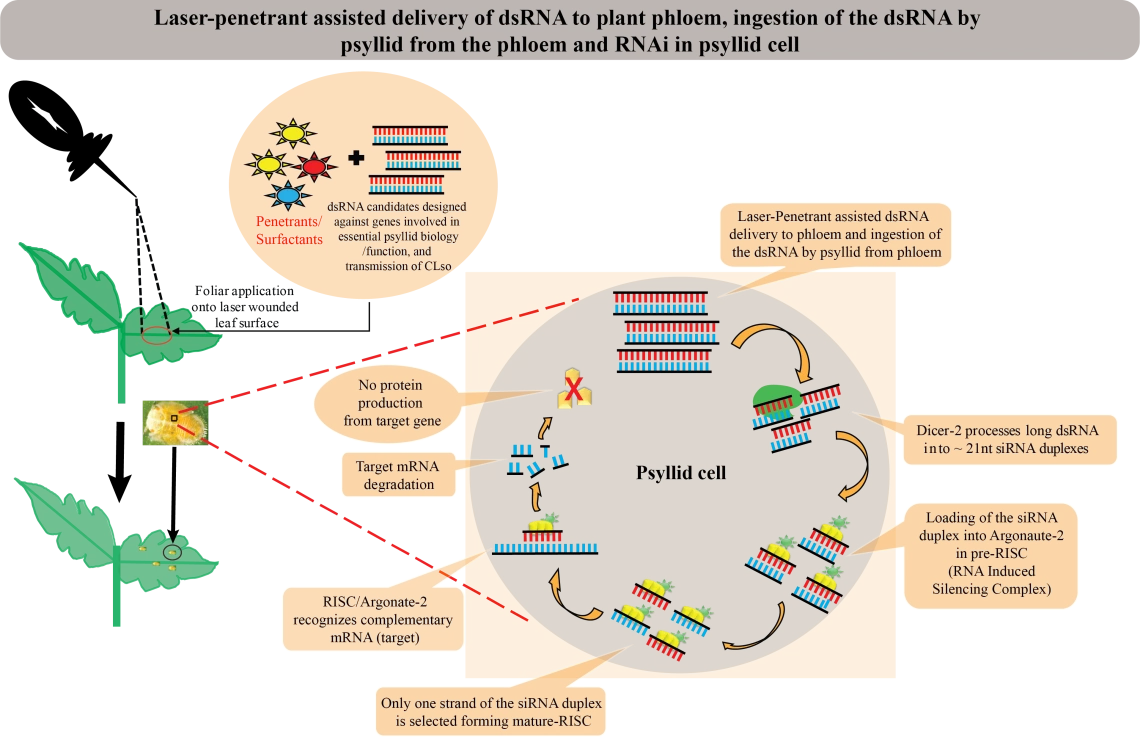
Project Goal
To develop new type of therapies, referred to as a ‘dsRNA biopesticides’, for their adoption as components of integrated pest and disease management (IPDM) practices to control citrus greening disease.
How do dsRNA biopesticides work?
- Biopesticide 'therapy' relies on the delivery of small dsRNA molecules that mimic psyllid gene sequences, to the plant vascular system from where psyllids ‘ingest’ the dsRNA during feeding. The dsRNA reduces psyllid gene expression, which interferes with expression of the protein encoded by the gene, through a multi-step pathway that results in a phenomenon referred to as ‘gene-silencing’.
- When the dsRNAs are applied to leaves (foliarly) or by other non-GMO modes or pathways into the vascular system of a citrus trees (phloem), including nanoparticles for example, or by root/soil drenching with dsRNA solution, the dsRNA enters the phloem and/or xylem, and is translocated systemically throughout the plant, including into the leaves, where psyllids feed on phloem (carbohydrates and amino acids), and at times on xylem contents (water).
- Once the dsRNA is ingested by psyllids during feeding, the dsRNA initiates a response that ‘knocks down’, or silences expression of the targeted psyllid gene that shares sequence homology with the dsRNA molecule.
- Ideally, when the target selected is responsive to robust knock-down, RNA-interference (RNAi) results in reduced gene expression which in turn reduces the amount of protein that would otherwise be synthesized to sub-functional levels.
- This is turn impairs important molecular and cellular functions in the target organism, resulting in disruption of physiological processes essential for psyllid livelihood, and ultimately, mortality.
- Important processes that may be affected by strategically silencing genes are digestion, molting, reproductive functions, metabolism, neurological functions, or disruption of specific behaviors required for survival and reproduction that result in impairment or death.
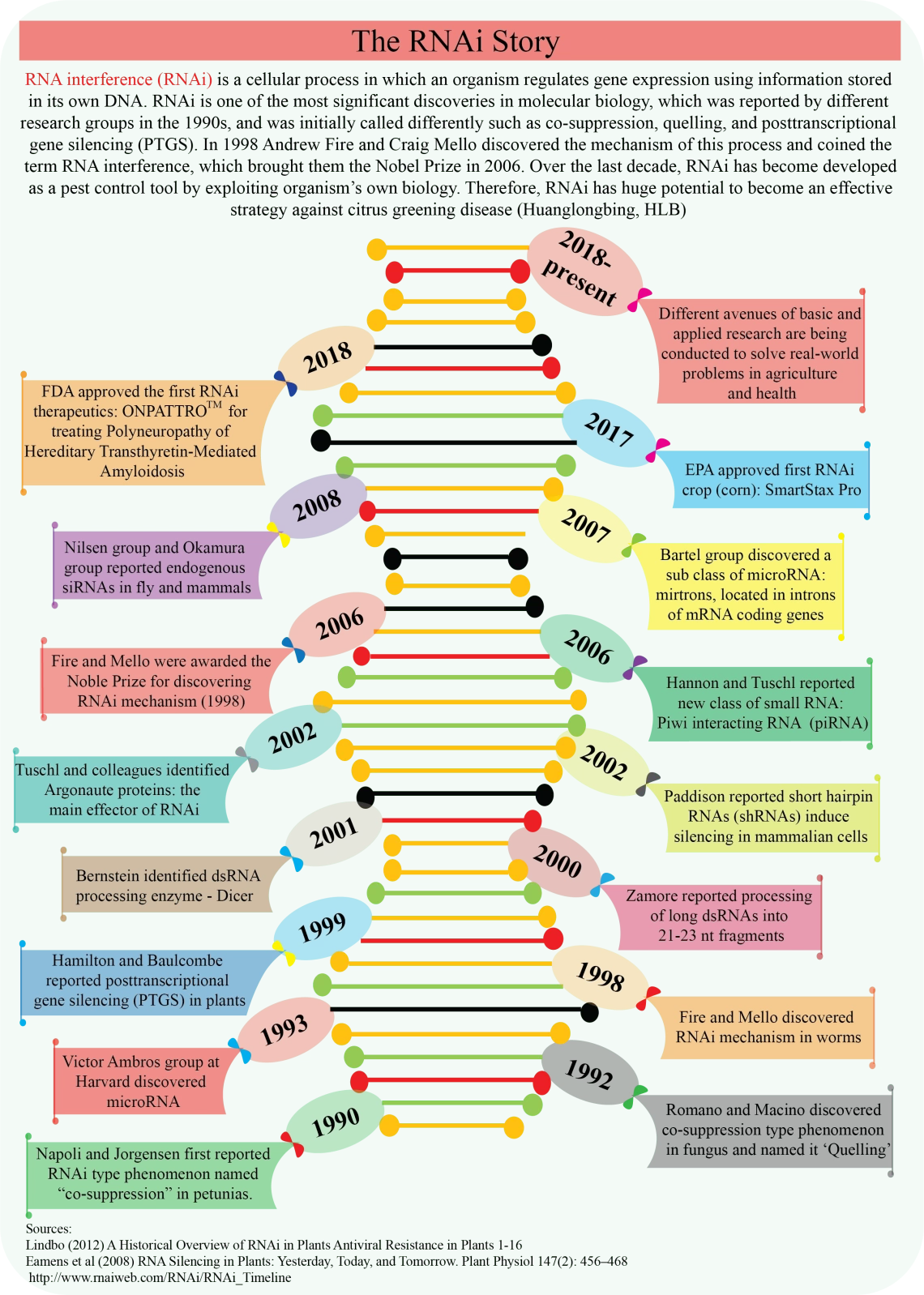
Brief history of RNA-interference (RNAi)
Objectives and Approaches Overview
Aims
To identify dsRNA biopesticidal activity with robust knockdown potential to:
- Achieve high mortality of psyllid immature instars or psyllid adults
- Abate Liberibacter transmission through knockdown of psyllid host genes exploited by Liberibacter for invasion, multiplication, and systemic infection
1. To design and deliver effective, psyllid target-specific dsRNAs that result in mortality of the psyllid vector, and/or interfere with psyllid-mediated CLas transmission, post-dsRNA ingestion.
2. The optimal effective amount of dsRNA required for knockdown and phenotype, respectively, will be determined for the potato psyllid (surrogate system for target selection) and the Asian citrus psyllid (biopesticidal knockdown and phenotype).
3. Different gene targets will be explored for lethality to immature and adult psyllids. Gene targets with potential to interfere with Liberibacter transmission (phenotype) will be evaluated in transmission bioassays.
4. Gene knockdown will be evaluated by real-time quantitative polymerase chain reaction (PCR) and/or drop-digital PCR. The mobility and persistence of selected dsRNAs in planta will be characterized.
5. This effort will couple translational research with Extension outreach and education pillars to improve HLB management.
Approaches
- Research teams at the University of Arizona (Dr. Judith Brown) and University of Florida (Dr. Jawwad Qureshi) will identify and validate psyllid gene targets that if knocked down, will result in psyllid mortality and/or reduced transmission competency. Studies in Arizona focus on the surrogate potato psyllid-CLso study system that allows rapid screening of targets in groups and singly. Gene targets that result in robust knockdown and discernable phenotype in potato psyllid, are evaluated for knockdown in the Asian citrus psyllid using dsRNAs homologous to the respective ACP genes. The strategy uses a strict GO / NO GO pipeline for target screening.
- The surrogate, potato/tomato psyllid-Ca. Liberibacter solanacearum study system has proven useful for ‘fast-track screening’ of potential target candidates because tomato plants are easier and faster to grow than citrus plants, thereby enabling screening of many gene targets with a short turn-round time, compared to ACP screens in citrus seedlings and trees.
- The extensive similarity of gene sequence and inferred functions in the genome of the two psyllids makes it possible to extrapolate between gene annotations and predicted functions in psyllid genome and transcriptome databases.
- Because the insect alimentary canal, or gut’ is the first point of contact of the dsRNA upon psyllid ingestion, the approach taken, herein, was to select genes expressed in the alimentary canal. These gene targets were identified in expression libraries (mRNA to cDNA) constructed from the extirpated potato psyllid and Asian citrus psyllid alimentary canal of psyllids infected by ‘Ca. Liberibacter solanacearum’ and ‘Ca. L. asiaticus’, respectively, and uninfected psyllids of each. By comparing the expression patterns between Liberibacter-infected and uninfected psyllids, psyllid host genes that responded to Liberibacter infection either by increased or decreased expression could be identified.
- Candidate gene targets are selected from among in silico gene annotations (transcripts, proteome), functional predictions, and reports in the scientific literature based on previously studied genes and gene families having broad functionality in multiple tissue types and organs across insects and other eukaryotic model and non-model organisms. Potential for non-target effects is considered in the pre-design and dsRNA design operations.
- Similar to other bacterial-host pathogen study systems, bacterial pathogens capitalize on their host’s functions to enable infection, invasion, reproduction, and spread to other tissues and organs. In this study system, Liberibacter infects its’ psyllid host, ultimately exiting the gut into the blood from where it invades the psyllid salivary glands. From here CLso or CLas are transmitted to the respective plant host, either tomato for CLso, or citrus, for CLas. Based on well-studied host-parasite/ host-pathogen systems, it can be hypothesized that Liberibacter alters the gene expression of the psyllid host to enable invasion, multiplication, and systemic infection. Analogously, Liberibacter infection of its plant host disrupts certain physiological functions to cause disease. Thus, CLso is the causal agent of vein-greening disease of tomato and of zebra chip disease of potato, while CLas causes citrus greening disease in grapefruit, oranges, and other citrus cultivars.
- The most promising targets identified define optimal delivery parameters for 'best dsRNA performers' based on dsRNA dose, efficacy, and persistence criteria. The long-term goal is to implement RNAi in citrus IPM programs to reduce psyllid vector numbers and lower CLas inoculum levels and deliver the product to plants using non-GMO approaches, including foliar laser-etching, virus-vector delivery, and other novel approaches.









